×
Network disruptions
We have been experiencing disruptions on our local network which has affected the stability of these web pages.
We have been working with IT support team to get this fixed as a matter of urgency and apologise for any inconvenience.
PDB Information
| PDB | 2QMC |
| Method | X-RAY DIFFRACTION |
| Host Organism | Escherichia coli |
| Gene Source | Helicobacter pylori |
| Primary Citation |
Characterization of Helicobacter pylori gamma-glutamyltranspeptidase reveals the molecular basis for substrate specificity and a critical role for the tyrosine 433-containing loop in catalysis.
Morrow, A.L., Williams, K., Sand, A., Boanca, G., Barycki, J.J.
Biochemistry
|
| Header | Transferase |
| Released | 2007-07-15 |
| Resolution | 1.550 |
| CATH Insert Date | 21 Feb, 2008 |
PDB Images (11)
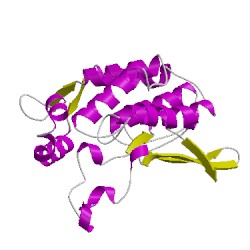
2qmcA01
CATH Domain 2qmcA01
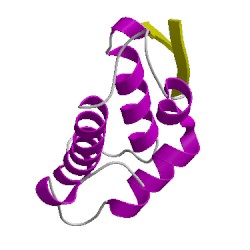
2qmcA02
CATH Domain 2qmcA02
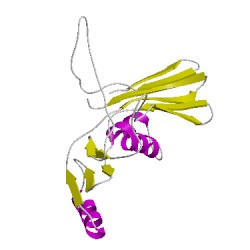
2qmcB00
CATH Domain 2qmcB00
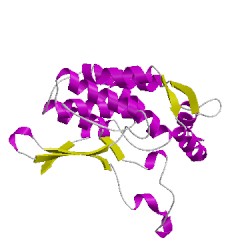
2qmcC01
CATH Domain 2qmcC01
2qmcC02
CATH Domain 2qmcC02
2qmcD00
CATH Domain 2qmcD00
PDB Chains (4)
CATH Domains (6)
UniProtKB Entries (4)
| Accession |
Gene ID |
Taxon |
Description |
| O25743 |
O25743_HELPY |
Helicobacter pylori 26695 |
Gamma-glutamyltranspeptidase (Ggt) |
| O25743 |
O25743_HELPY |
Helicobacter pylori 26695 |
Gamma-glutamyltranspeptidase (Ggt) |
| O25743 |
O25743_HELPY |
Helicobacter pylori 26695 |
Gamma-glutamyltranspeptidase (Ggt) |
| O25743 |
O25743_HELPY |
Helicobacter pylori 26695 |
Gamma-glutamyltranspeptidase (Ggt) |
