CATH Documentation
Jelly Roll
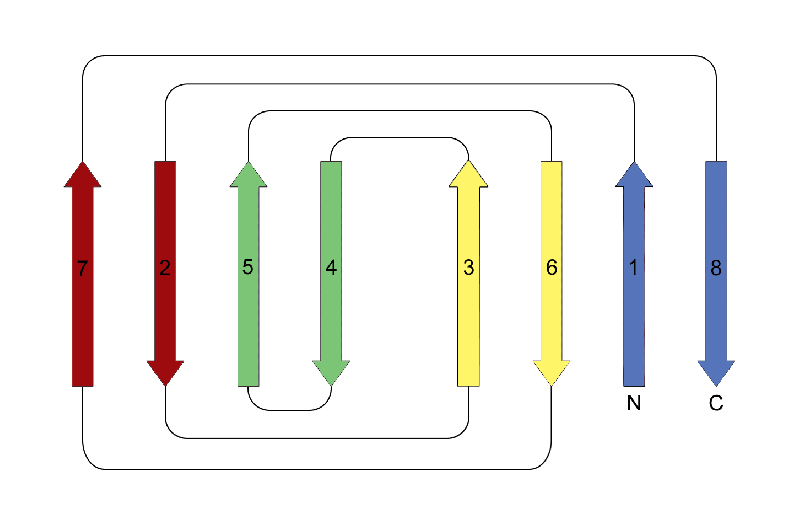
This is a fold topology that classically consists of four Greek key motifs that adopt an eight-stranded beta sandwich structure. In this fold the hydrogen bonding pattern between adjacent strands is broken in two places, and as a consequence the structure comprises two four-stranded beta sheets. Both sheets are purely antiparallel, with strands adjacent in sequence appearing in different sheets with the exception of the fourth and fifth strands, which are in the same sheet. This leads to a structure with only one hairpin; all other beta-beta connections are arches. When the structure is depicted in two-dimensions the origin of its name is apparent: the structure resembles a jelly roll (Swiss roll).
In theory there are four types of jellyroll folds: two have a right-handed superhelical twist, and two have a left handed twist. However, as with the beta-alpha-beta motif , only right-handed Greek key and jellyroll structures are actually observed in globular proteins. In practise, the jelly roll may be varied by the addition or deletion of secondary structure elements.
The example shown is a the carboxy-terminal domain of phosphomannose isomerase (CATH domain ID 1pmiA03).
References
Stirk HJ, Woolfson DN, Hutchinson EG, Thornton JM
FEBS Lett308p1-3(1992 Aug 10)